
Introduction
In a world where Instagram filters, TikTok trends, and instant online shopping make cosmetic change seem fast and harmless, millions pursue artificial beauty through injectables, implants, skin-lightening creams, non-surgical lifts, and cheap cosmetics. While many procedures deliver satisfying results, the rise in counterfeit products, unregulated treatments, and social pressures has exposed users to serious medical, psychological, and public-health risks. This article explains the health risks, reviews recent scientific and regulatory findings from Asia, America, and Europe, outlines the psychological impact of cosmetic culture, and gives practical, evidence-based guidance to stay safe. Key points are highlighted for quick reading, a reference table summarizes typical complications, and a list of up-to-date studies and reputable sources is provided at the end.
Key points
- Fillers and injectables can cause mild side effects — swelling, bruising — but also serious complications such as infections, granulomas, vascular occlusion, necrosis, and even hospitalization. PMC+1
- Breast implants carry documented risks (capsular contracture, rupture, implant illness) and have been associated with a rare lymphoma (BIA-ALCL); regulators in the U.S. and Europe monitor these risks closely. U.S. Food and Drug Administration+1
- Skin-lightening creams containing mercury or unregulated steroids cause systemic toxicity — kidney, neurological, and dermatologic damage — and are a global public-health concern. Organisation mondiale de la santé+1
- Counterfeit or mislabelled cosmetics and treatments sold online often contain toxic or contaminated ingredients and are a growing international problem. www.personalcareinsights.com+1
- Psychological harms — body dissatisfaction, worsening mood disorders, and social-media-driven expectations — are significant drivers of cosmetic procedures and often go unaddressed in preoperative evaluation. PMC+1

The medical risks of artificial beauty
Injectables, fillers, and “quick” non-surgical procedures
Dermal fillers (hyaluronic acid, biostimulatory fillers), botulinum toxin, and off-label injectable materials are widely used. Systematic reviews and hospital case series report a spectrum of complications: temporary injection-site reactions (pain, swelling, bruising), but also delayed granulomas, soft-tissue infections, necrosis from vascular compromise, blindness (very rare), and admissions for severe cellulitis or abscess. A 2024 review and 2024 hospital analysis documented that a meaningful subset of complications require hospitalization and aggressive treatment. Practitioners and patients must recognize that “minimally invasive” does not mean “risk-free.” PMC+1
Surgical implants and body contouring
Breast implants and surgical body-contouring procedures have long-term complication profiles: capsular contracture, rupture, chronic pain, and, in rare cases, breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL). Regulatory agencies such as the FDA maintain databases and issue guidance for clinicians and patients regarding implant surveillance and reporting. Recent reviews emphasize careful informed consent and long-term follow-up. U.S. Food and Drug Administration+1
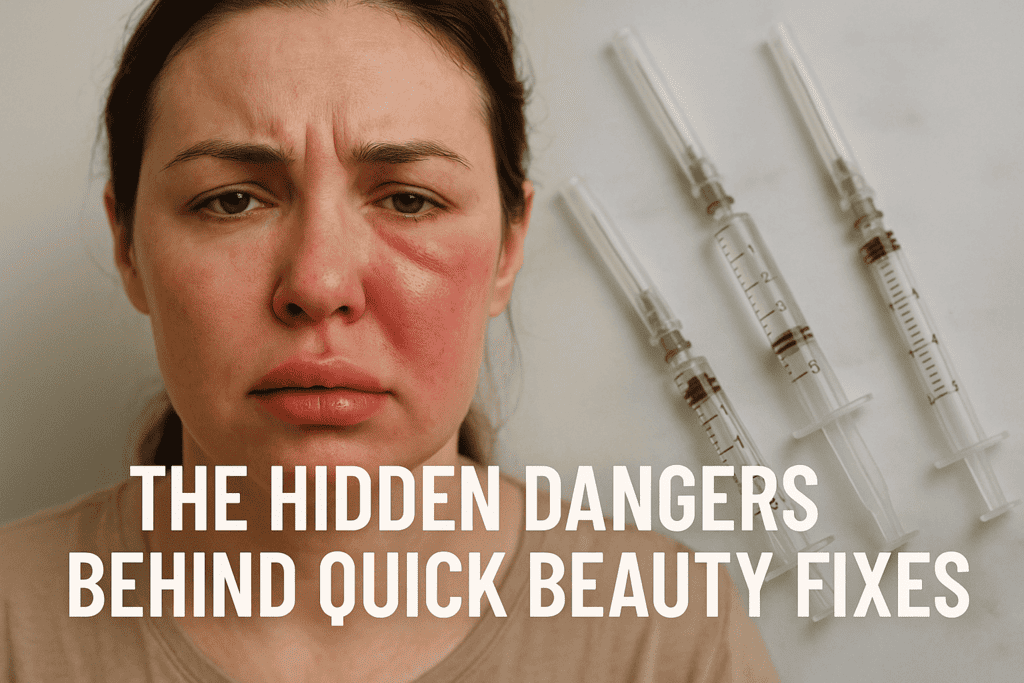
Dangerous, unapproved, or illicit substances
Injecting industrial silicone or purchasing “liquid BBL” solutions from unregulated providers has led to severe complications including embolism, multiorgan failure, and deaths. Similarly, counterfeit or home-made fillers have led to outbreaks of severe infections and granulomatous reactions. Public health warnings consistently emphasize that only approved materials administered by trained clinicians should be used. Allure+1
Skin-lightening and cosmetic creams
Skin-lightening products remain popular in many regions. However, many illicit creams contain mercury or high-dose corticosteroids. Mercury exposure via topical products causes neurological, renal, and dermatologic damage; steroid contamination can produce skin atrophy, infections, and systemic hormonal effects. The World Health Organization and peer-reviewed systematic reviews document these harms and advocate for regulatory action. Organisation mondiale de la santé+1

Psychological and social harms
Cosmetic procedures are not performed in a psychological vacuum. Multiple studies and reviews (Asia, Europe, North America) show that social-media exposure, photo-editing apps, and cultural norms increase appearance dissatisfaction, sometimes fueling repeated or unnecessary procedures. For some patients, cosmetic interventions improve self-esteem and quality of life; for others, preexisting mood disorders, body dysmorphic disorder (BDD), or unrealistic expectations lead to poorer outcomes. Clinicians should screen for psychological vulnerability before treatment. PMC+1
Important psychological drivers :
- Social comparison driven by filtered images and influencer norms. New York Post
- Perfectionism and impulsive decision-making in younger patients. ScienceDirect
- Reinforcement loops: small corrections often prompt patients to chase further “perfections,” increasing risk exposure.
Public-health and supply-chain issues
Global trade in counterfeit cosmetics and illicitly manufactured aesthetic products is increasing. Reports from the OECD, regulatory agencies, and investigative journalism show that online marketplaces can distribute fake, contaminated, or mislabelled products across borders — a hazard for consumers in Asia, Europe, Africa, and the Americas. This trend underlines the importance of supply-chain vigilance and buying only from regulated pharmacies or licensed clinics. www.personalcareinsights.com+1

Table : Common procedures and associated risks (summary)
| Procedure / product | Typical mild effects | Serious risks | Evidence / guidance |
|---|---|---|---|
| Dermal fillers (HA, biostimulants) | Swelling, bruising, nodules | Vascular occlusion, infection, necrosis, granulomas | Systematic reviews; hospital case series. PMC+1 |
| Botulinum toxin | Temporary weakness, bruising | Spread of toxin (rare), airway compromise (very rare) | Reviews and safety studies. ScienceDirect |
| Breast implants | Pain, asymmetry, capsular contracture | Rupture, BIA-ALCL (rare), systemic symptoms | FDA reports; surgical reviews. U.S. Food and Drug Administration+1 |
| Skin-lightening creams (illegal) | Irritation, dermatitis | Mercury poisoning, kidney damage, steroid effects | WHO action plan; systematic reviews. Organisation mondiale de la santé+1 |
| Counterfeit cosmetics | Contact dermatitis | Severe systemic toxicity, infections | OECD reports; investigative findings. www.personalcareinsights.com+1 |
How to reduce risk — evidence-based recommendations
- Choose licensed, properly trained clinicians. Board certification, clinic accreditation, and documented experience reduce complications. Ask for before/after photos and complication management plans. (Supported by clinical guidance and case series.) PMC+1
- Verify product approvals and traceability. Use only products that are approved by your national regulator (FDA, EMA, NMPA, etc.) or supplied through licensed pharmacies. Avoid bargain offers from unverified online sellers. U.S. Food and Drug Administration+1
- Screen for psychological vulnerability. Before irreversible procedures, clinicians should screen for mood disorders or BDD and refer for psychological evaluation when indicated. Evidence shows preexisting mental health conditions predict poorer satisfaction and increased regret. PMC+1
- Avoid unregulated “DIY” or off-label injections. Industrial silicone and black-market fillers have caused fatal complications. Only FDA/EMA-approved substances should be used by qualified specialists. Allure
- Be cautious with skin-lightening products. Check ingredient lists; avoid products with mercury, high-dose steroids, or unspecified “herbal” blends. If in doubt, consult a dermatologist. WHO guidance emphasizes elimination of mercury-containing products. Organisation mondiale de la santé+1
- Report adverse events. If you experience an unexpected complication, seek emergency medical care and report the event to public-health or regulatory authorities — this improves surveillance and protects others. U.S. Food and Drug Administration

A short note on regulation and research from different regions
Recent scientific and regulatory literature from Asia, Europe, and North America consistently highlight the same themes: rising complication rates tied to the beauty industry’s rapid growth, the public-health risks posed by counterfeit or mercury-containing products, and the psychological drivers intensified by social media. National agencies (FDA in the U.S., national health authorities in Europe and Asia) have issued warnings and guidance while academic centers have published reviews documenting both clinical complications and psychosocial outcomes. These convergent findings point to global patterns requiring coordinated response. PMC+2Organisation mondiale de la santé+2
Conclusion
Artificial beauty — from a tiny filler to a surgical implant or a widely advertised cream — can bring real benefits when used responsibly. However, the hidden dangers are real: medical complications (sometimes severe), systemic toxicity from unregulated products, psychological harm driven by unrealistic standards, and the dangers of counterfeit supply chains. The best defense is informed caution: use licensed providers, verify product approvals, screen for psychological vulnerability, avoid black-market or DIY treatments, and report adverse events. Policymakers, clinicians, and consumers must work together to prioritize safety and transparency in the global cosmetic marketplace.
Selected references (studies, reviews, and authoritative sources)
- Hong GW, et al. Review of the Adverse Effects Associated with Dermal Fillers and Injectables (2024). PubMed Central. PMC
- Ehsani A, et al. Analysis of Hospitalization Cases for Filler Injection Complications (2024). PubMed Central. PMC
- Jones HE. The Psychological Impact of Aesthetic Surgery: A Mini-Review (2022). PubMed Central. PMC
- U.S. Food & Drug Administration (FDA). Risks and Complications of Breast Implants; BIA-ALCL reporting. (Updated 2023–2025). U.S. Food and Drug Administration+1
- World Health Organization (WHO). Elimination of mercury-containing skin lightening products. Organisation mondiale de la santé
- Bastiansz A, et al. Systematic Review of Mercury Exposures from Skin-Lightening Products (2022). PubMed Central. PMC
- Pollock S, The dark side of skin lightening: An international perspective (2020). PubMed Central. PMC
- OECD / investigative reporting and trade analysis on counterfeit cosmetics (2025). Coverage on counterfeit trade and public-health risks. www.personalcareinsights.com
- Di Santis ÉP, Adverse effects of the aesthetic use of botulinum toxin and other injectables (2024). ScienceDirect. ScienceDirect
- Janovskiene A, Safety and Potential Complications of Facial Wrinkle Injections (2024). MDPI. MDPI





